Raw read quality control with nf-core/rnaseq
- Understand how to apply parameters to an nf-core pipeline to cusomise its execution
- Understand the fastq file format
- Learn how to interpret a FastQC report for RNAseq data
- Learn about the benefits and drawbacks of read trimming for RNAseq-DE
We’re starting at the first bioinformatics stage of the RNAseq workflow, specifically with raw data QC step (red box below). When your data is sequenced, it will be output in the fastq format by the sequencing machine.
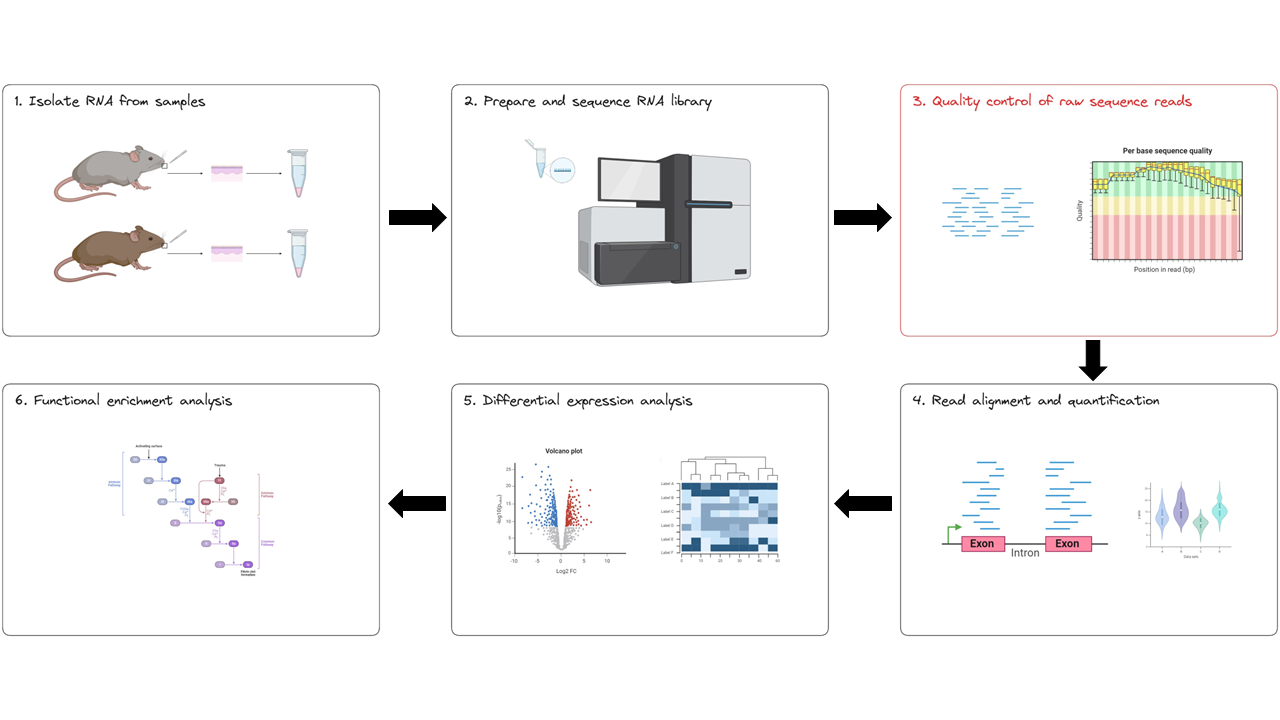
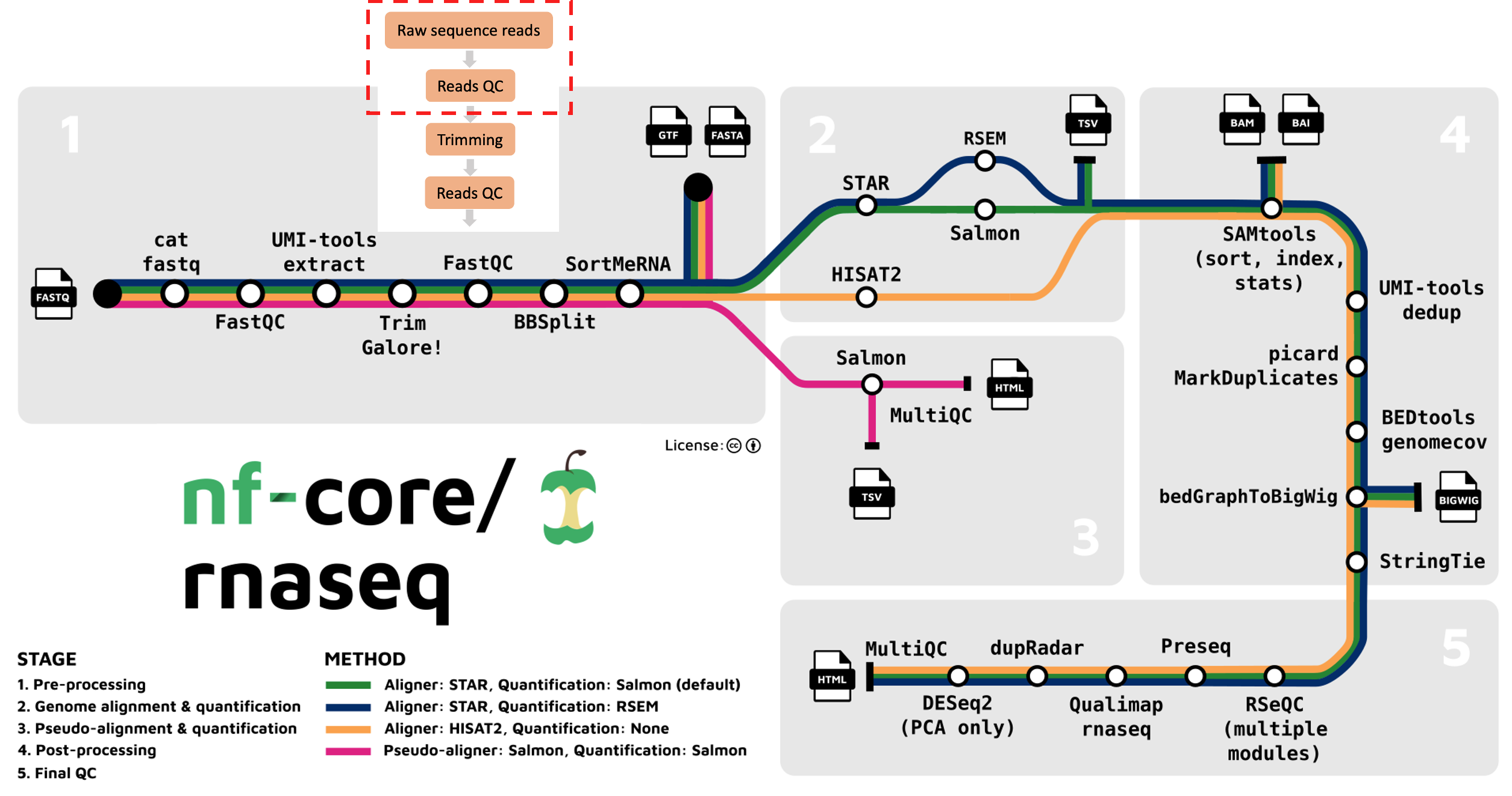
The nf-core/rnaseq pipeline uses FastQC to evaluate fastq files. FastQC provides a set of metrics which we can use to get a sense of whether our raw data has any problems that might impact our ability to analyse it downstream. This step corresponds to the first part (red box) of the nf-core/rnaseq pipeline.
When your data is sequenced, it will be output in the fastq format by the sequencing machine. Fastq is a text-based format for storing both a biological sequence (usually nucleotide sequence) and it’s corresponding quality score. Each entry in a fastq file will consist of 4 lines:
- A sequence identifier (label)
- The nucleotide sequence
- A separator line, usually just a plus (+) sign
- The base call quality (phred) score per nucleotide.
Phred quality scores are used to indicate the quality of a base call. The Phred score corresponds to the probability that the base was called correctly. Take a look at GATK’s explanation of Phred scores for more information.
1.2.1 Run the qc command
In any bioinformatics experiment, it is crucial that you perform quality control (QC) on your data before you process it. Why?
- Identify issues that may interfere with analysis and interpretation
- Detect biases which may have been introduced during library preparation or sequencing
nf-core/rnaseq provides users the option to run FastQC and read trimming only. We will do this use the --skip_alignment flag/parameter. Using this flag will run 3 steps in the workflow:
- Check quality of raw sequence reads for each sample with FastQC
- Perform raw read trimming with trim galore!
- Check quality of trimmed reads for each sample with FastQC
- Output a MultiQC report
nf-core/rnaseq developers have only recently added this QC-only functionality into their pipeline code. Up until June 2023, you had to run the pipeline in full before you could examine the raw sequence QC outputs. After a number of users petitioned the developers (see this Github issue for our pleas), the developers added the --skip_alignment feature.
➤ Run the following command to perform the QC steps above:
nextflow run nf-core-rnaseq_3.12.0/3_12_0/main.nf \
--input ~/Data/samplesheet.csv \
--outdir WBS-mouse-QC \
--fasta ~/Data/mm10_reference/mm10_chr18.fa \
--gtf ~/Data/mm10_reference/mm10_chr18.gtf \
--star_index ~/Data/mm10_reference/STAR \
--salmon_index ~/Data/mm10_reference/salmon-index \
-profile singularity \
--skip_alignment \
--max_memory '6.GB' \
--max_cpus 2Once the pipeline has been executed with the run command, you will see a message printed to the screen. This message contains a summary of the parameters you have used to execute the workflow.
In addition to this you will see a number of processes spawn out from the workflow. Your screen will progressively update as these tasks are completed. Once they have all finished, a completion message will be printed to your screen:
-[nf-core/rnaseq] Pipeline completed successfully -
Completed at: 29-Sep-2023 09:17:28
Duration : 2m 31s
CPU hours : 0.1
Succeeded : 191.2.2 Evaluate the data quality
➤ Take a look at the output that is produced by your qc run:
ls -lh /data/Day-1/WBS-mouse-QCtotal 24K
drwxrwxr-x 6 training training 4.0K Sep 29 09:38 .
drwxrwxr-x 10 training training 4.0K Sep 29 05:51 ..
drwxrwxr-x 2 training training 4.0K Sep 29 09:37 fastqc
drwxrwxr-x 4 training training 4.0K Sep 29 09:38 multiqc
drwxrwxr-x 2 training training 4.0K Sep 29 09:38 pipeline_info
drwxrwxr-x 3 training training 4.0K Sep 29 09:37 trimgaloreWe have 4 directories within our output directory. Using the File Explorer on VS code, open the WBS-mouse-QC directory and download the multiqc report multiqc/multiqc_report.html to your computer. This MultiQC report aggregates raw and trimmed data FastQC reports for each sample. Open this file in your web browser using Live Server and answer the questions below.
- Open your file explorer panel in VS Code
- Open the WBS-mouse-QC directory
- Open the multiqc directory
- Right click on multiqc_report.html
- Select open with live server
If Live Server doesn’t work, right click on multiqc_report.html and select download.
Looking at the Fastqc (raw) section of the MultiQC report:
- How many unique and duplicate reads are in
SRR3473989.fastq? - What might be a reason for the high proportion of duplicate reads in this dataset?
- Take a look at the Per Base Sequence Content plot for all samples. Which part of the reads tend to have worse per base sequence quality?
- Do you think this dataset is of ‘good’ quality? Why or why not?
- Do you have any suggestions to improve the quality of our raw reads?
- 35,519 and 24,368
- RNAseq only captures transcripts, therefore the chances of observing duplicate reads is elevated.
- Reads which tend to have worse per base sequence quality are towards the right hand side (3’ end).
- The color coding separates out regions of good quality (Red PhredQ > 28) from the rest. Overall yes, as most of the regions of the reads show quality values in red.
- We can trim the bases towards the 3’-end and hope to improve the overall read-quality. But trimming by quality for RNA-seq data has its pros and cons.
FastQC was designed for whole genome sequencing (WGS) and not RNAseq experiments. With this knowledge, can you identify why some categories might have been marked as “fail” or “warn” by looking at the HTML reports?
- Per-base sequence content fails. Per sequence GC content, sequence duplication levels, and overrepresented sequences return warnings.
- Per-base sequence content fails because we always see bias at the start of RNA-seq reads, which tells us the random priming is not ‘truly random’. See here for a nice explanation of this.
- Per sequence GC content, sequence duplication levels, and overrepresented sequences return warnings are received for this same reason.
- Given there is much less RNA sequence then DNA in our bodies, we don’t observe these biases in WGS.
- By chance, RNA will be fragmented at the same spot and sequenced multiple times. For DNA, the purpose of these plots is to check for technical bias (optical duplicates - when the sequencer reads the same strand multiple times).
1.2.3 Read trimming
Trimming is sometimes performed to improve the quality of the raw data and potentially improve its mapability when it is being aligned to a reference genome. There are several ways to perform trimming:
- Removal of poor quality reads or bases (e.g. ends of reads)
- Removal of adapter sequences
- Removal of polyA tails
The nf-core/rnaseq pipeline uses Trim Galore for read quality trimming. It is able to perform quality-based removal of low-quality bases and adapter trimming. Given trimming can result in some reads being significantly shortened (sometimes to 0bp!), Trim Galore will filter reads that are too short to be used in downstream processes like read alignment. We are still looking at the outputs from the first part of the nf-core/rnaseq workflow below (red box).
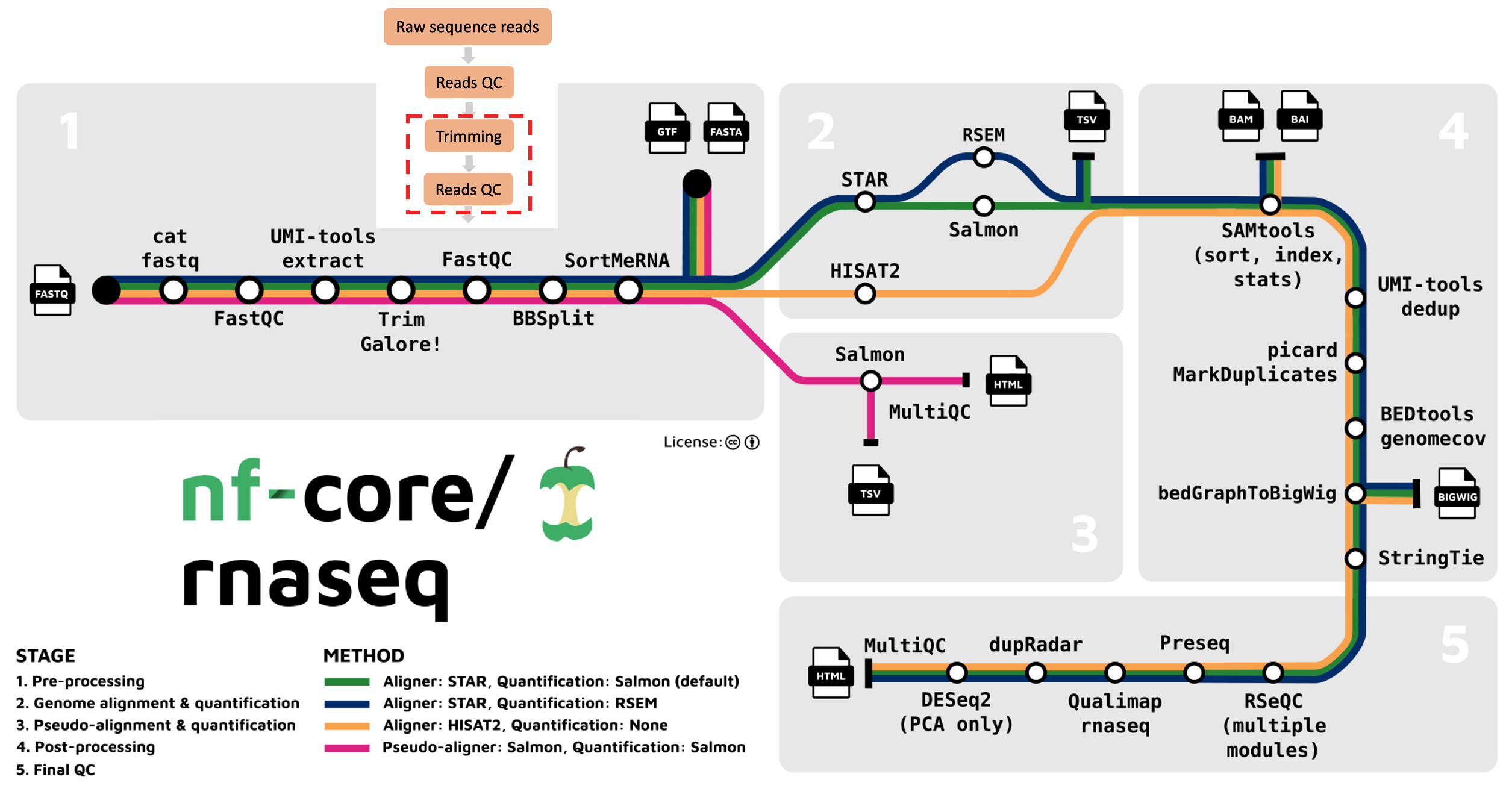
Does trimming help?
Read trimming is not always a necessary step when processing next generation sequencing (NGS) data. These days, NGS data is of a very high quality and the tools we use to perform processes like read mapping are capable of handling poor quality reads and adapter sequences.
While the trimming adapter sequences has been shown to increase the quality of RNA-seq data (Dozmorov et al., 2015), other studies have shown that trimming of poor quality reads can effect gene expression estimates (Williams at el., 2016).
When making the decision to trim your reads for differential expression RNAseq studies, we suggest following the recommendations of the read alignment tool you’ll be using. We will explore trimming outcomes.
➤ Open your Nimbus terminal again to do the challenge exercise below. Navigate to the results directory either on the command line or using your VS Code File Explorer and answer the following questions:
cd ~/Day-1/WBS-mouse-QC- Which tool does nfcore-rnaseq use for read-trimming?
- Which tool did you use to generate quality reports before and after trimming?
- What effect did trimming have on SRR3473989.fastq?
- Trim galore.
- FastQC generates
.htmlreports. - Total number of sequences have reduced after trimming. Read length is now 21 - 101. Per base sequence quality now mostly in the green.
Open trimgalore report by running:
cat WBS-mouse-QC/trimgalore/SRR3473984.fastq.gz_trimming_report.txt- Quality control is fundamental in RNAseq experiments to identify any issues that might skew analysis and interpretation.
- The Fastq file format is the primary format used for raw sequencing data. Each entry in a Fastq file has a sequence identifier, the nucleotide sequence itself, a separator, and a base call quality score (Phred score) for each nucleotide.
- Read trimming is a process where certain portions of the raw sequence data are removed to enhance its quality and improve its mappability during alignment. The decision to trim reads in an RNAseq experiment should be based on the recommendations of the alignment tool used and the quality of your data.